











In the United States alone, approximately 50 million people are affected by an autoimmune disease, with some studies suggesting that this figure is increasing by 3 to 12% annually.4 For common autoimmune diseases such as Systemic Lupus Erythematosus and Sjögren’s Syndrome, which affect females significantly more than males, up to 3 and 2.4 million adult women are estimated to be affected by these diseases, respectively.5,6 Unfortunately for these women, treatment options are extremely limited. Only one targeted therapy has been commercially approved for Lupus, and for Sjögren’s, there is none.
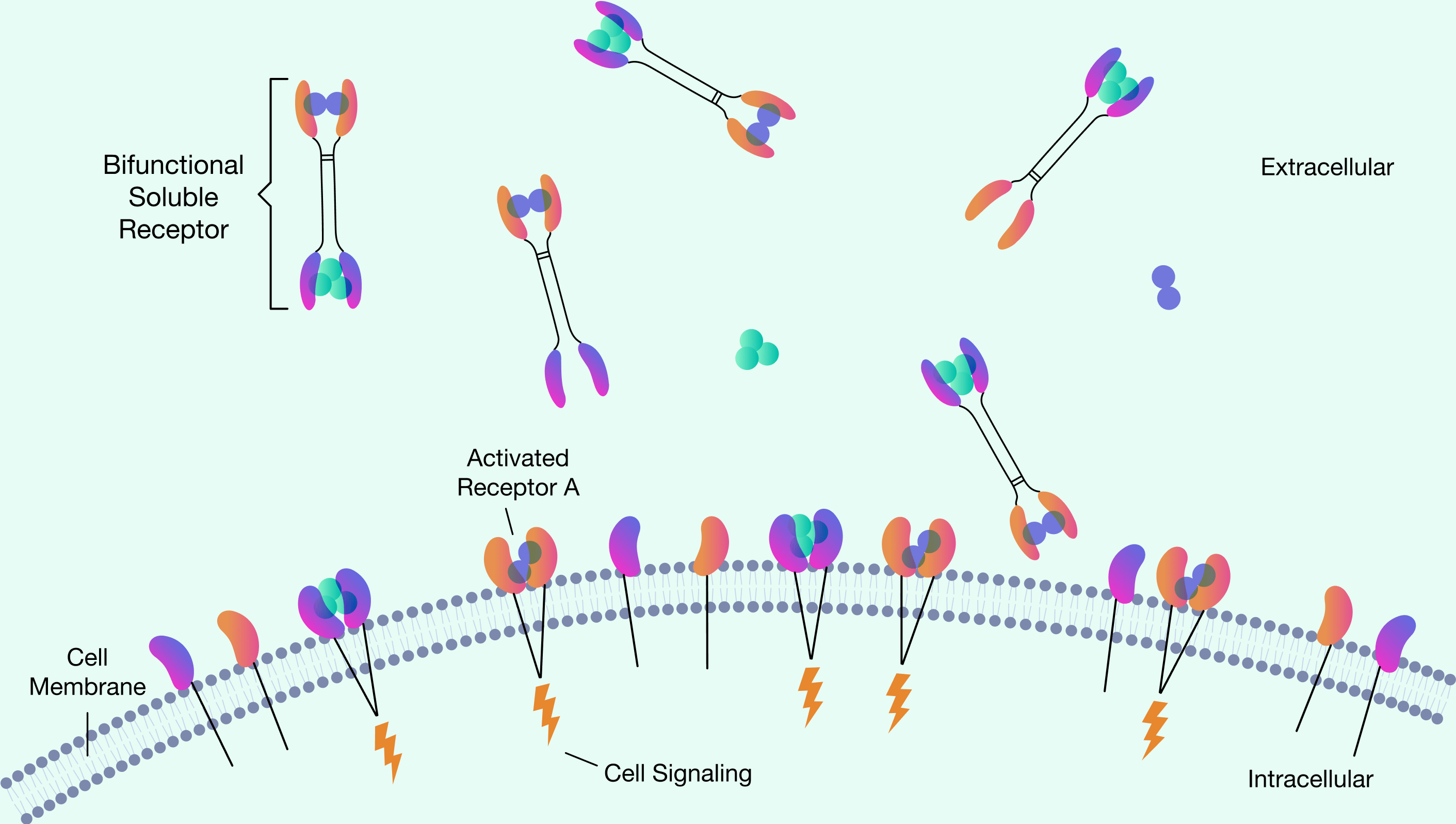
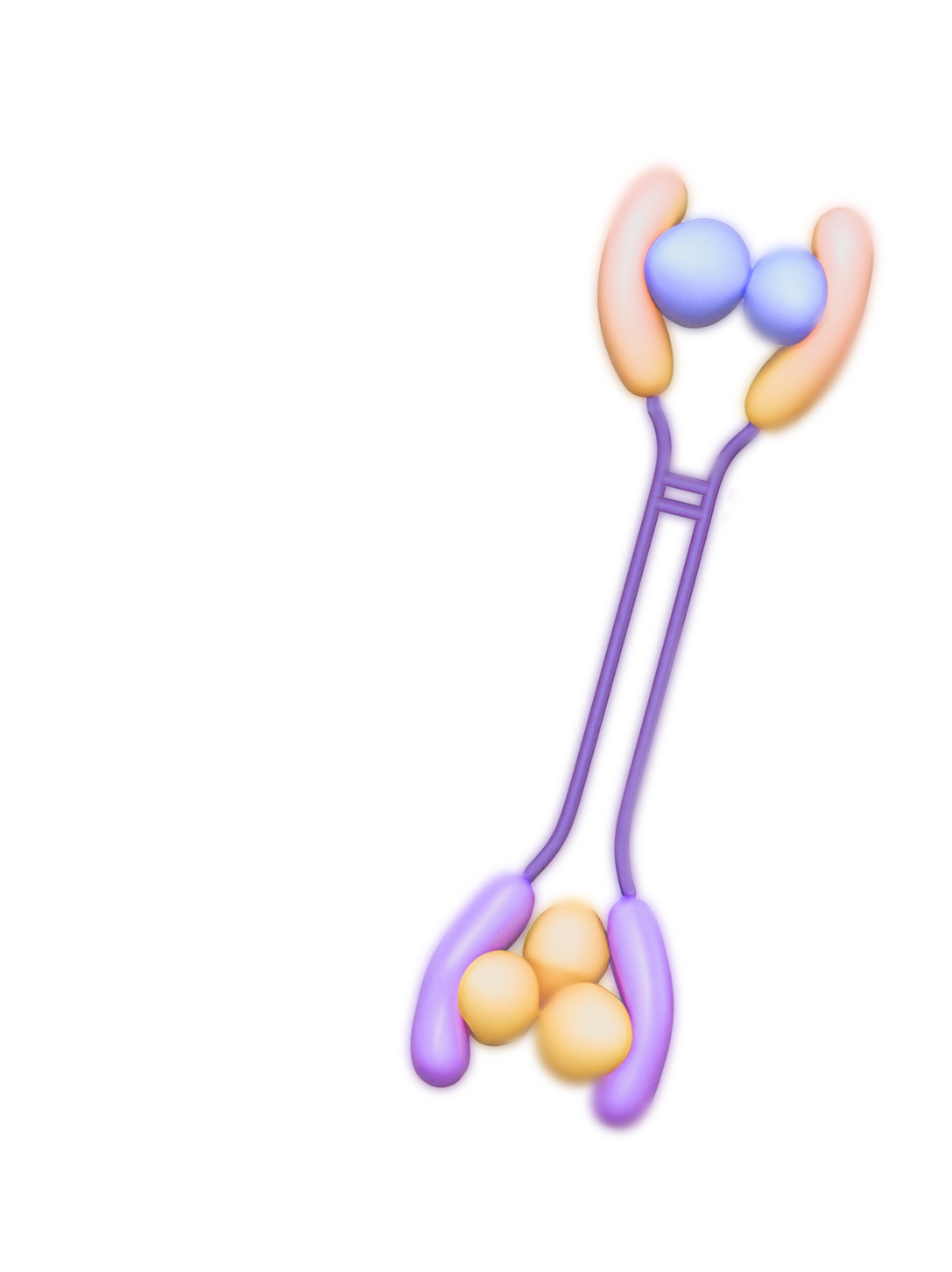
Fab Biopharma has detailed operational knowledge for generating preclinical data which will support IND submissions
for initiation of clinical studies on bispecific receptor fusion drugs in separate disease indications.




The female sex is associated with an enhanced function of both innate and adaptive immune pathways, resulting in better immune defense and increased autoimmune disease susceptibility. The IncRNA molecule Xist, present in every female cell and central to the proper regulation of the X chromosomes, can sometimes inadvertently create protein complexes strongly associated with developing an autoimmune response.7
Fab Biopharma is pioneering drug discovery from a female perspective by actively prioritizing female patient samples and animal models and designing clinical trials with predominantly female subjects for diseases prevalent primarily in women. It is also undertaking additional R&D projects exploring genetic markers, including the Xist ribonucleoprotein complex.
Fab’s goal is to reduce sex-based scientific bias including in formative cellular biology studies and landmark clinical trials. From use of predominantly male cell lines and male animal models to the historic limiting of clinical trial participation to primarily males, sex-based bias has greatly contributed to the lack of understanding of and efficacious treatments for predominantly female autoimmune diseases.8




Chia Chia Sun is the Chief Commercial Officer at FAB Biopharma, leveraging over 20 years of experience in women’s health, personal care, and biotechnology. Previously the CEO of Damiva Inc., she has a robust background in clinical trial management and corporate finance. Chia Chia has served in leadership roles at Azanta and TenX Biopharma. She holds an MBA in Corporate Finance from the University of Toronto’s Rotman School of Management and an MSc in Experimental Medicine and Bioethics from McGill University, bringing multifaceted expertise to her commercial strategies.

Gardiner Smith is the CEO of Fab Biopharma, leading the company since January 2021. With over two decades of experience in the biopharma industry, he has previously held executive roles at Glaxo, Human Genome Sciences, Memory Pharma, Aspreva and AgeneBio. Gardiner holds a Bachelor’s degree in Chemistry from the University of North Carolina at Chapel Hill and a JD from North Carolina Central University. He is a member of the U.S. Patent Bar.

Bill Freimuth is the Head of Clinical and Regulatory at FAB Biopharma, bringing 28 years of experience in translational medicine and clinical development for autoimmune and infectious diseases. He has led pivotal clinical trials and regulatory submissions for therapies targeting HIV and SLE. With a PhD in Immunology and an MD specializing in Internal Medicine and Rheumatology, Bill’s extensive background includes senior roles at Pfizer and Human Genome Sciences, where he contributed to significant therapeutic advancements and regulatory approvals.

Reiner Gentz is the Chief Scientific Officer at FAB Biopharma, with over 25 years of expertise in protein development, cGMP manufacturing, and regulatory compliance. He holds a PhD in Molecular Biology and has a distinguished career developing therapeutic proteins, antibodies, and vaccines. Reiner has held senior positions at Human Genome Sciences and founded Gentz Biopharmaceutical Consulting LLC. He has played key roles in developing groundbreaking biologics, including the first FDA-approved drug for lupus, Benlysta®, and has extensive experience designing advanced biopharmaceutical facilities.

.png)



.png)
_bb_grain (2).png)
_bb_grain (1).png)
_bb_grain.png)
.png)
.png)
.png)